Measurements in Physics - Topic 1.1
Bite-sized Measurements Study Notes for IB Physics HL/SL
Share on facebook
Facebook
Share on twitter
Twitter
Share on linkedin
LinkedIn
Share on reddit
Reddit
Share on email
Email
Share on whatsapp
WhatsApp
Table of Contents
Key points
- The fundamental units of the SI system are: ampere, Kelvin, kilogram, meter, mole, and second (along with candela which is not needed for IB Physics)
- Always convert to these units.
- Numbers that are very big or very small can be expressed in powers of 10 (scientific notation)
- Become familiar with the corresponding metric prefixes.
- There are seven fundamental units of the SI system, six of which are used for the IB.
- Luminous Intensity is measured in candela (cd) but is not used in the IB curriculum
- Whenever working with any relevant quantities, convert all given values to these units.
| Quantity | Name | Symbol |
|---|---|---|
| Length | Meter | m |
| Mass | Kilogram | kg |
| Time | Second | s |
| Electric Current | Ampere | A |
| Thermodynamic Temperature | Kelvin | K |
| Amount of Substance | Mole | mol |
| Power | Prefix | Symbol |
|---|---|---|
| 10-12 | Pico- | p |
| 10-9 | Nano- | n |
| 10-6 | Micro- | µ |
| 10-3 | Milli- | m |
| 10-2 | Centi- | c |
| 103 | Kilo- | k |
| 106 | Mega- | M |
| 109 | Giga- | G |
- Small or large quantities can be expressed in terms of units that are related to the basic ones by powers of 10
- This is known as scientific notation
- Each power has a prefix associated with it shown to the table on the left.
- mirco, milli, Kilo, etc. are known as metric prefixes.
- Become familiar with their names, symbols, and corresponding powers.
- mirco, milli, Kilo, etc. are known as metric prefixes.
- Expressing a quantity as a plain power of 10 gives what is called the order of magnitude of that quantity
- This can be used to find the ratio between two different quantities
Key point
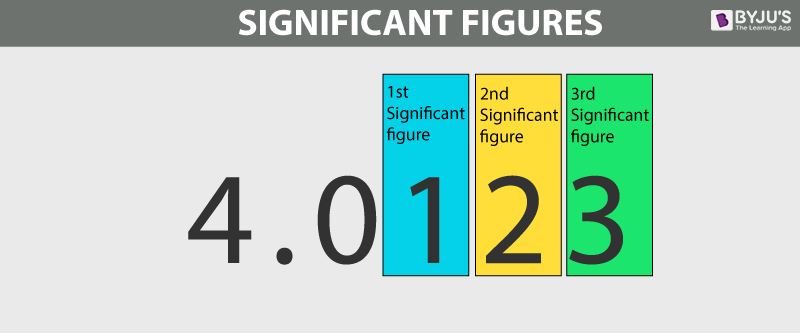
- Significant Figures are used to indicate the quantity of something to a reliable and necessary amount of digits (A more precise way of rounding).
- If not specified, always give your answer on IB exams to 3 significant figures.
The number of digits used to express a number carries information about how precisely the number is known
- For example, a stopwatch reading of 3.2s is less precise than a reading of 3.22 seconds
For some values, a number should be more precise (more significant figures), and for others, a number should be less precise (less significant figures)
How many significant figures are used in IB Physics? If not specified, always give your answer on IB exams to 3 significant figures.
There are certain rules which need to be followed to measure the significant figures of a number
- All non-zero (1-9) digits are significant.
- Zeroes between non-zero digits are significant.
- A trailing zero or final zero in the decimal portion only are significant.
A nice trick to help is the Pacific-Atlantic Rule:
- The rule states that if a decimal point is Absent, then the zeroes on the Atlantic/right side are insignificant. If a decimal point is present, then the zeroes on the Pacific/left side are insignificant.
Subscribe to the Inertia Newsletter
IB News, Covid-19 Updates, Deadlines, Tips and Tricks, and Hundreds of Free Resources are Awaiting You!
Features
- Study Notes
- Thousands of IB Questions
- Detailed Answers
- Ask-A-Question System