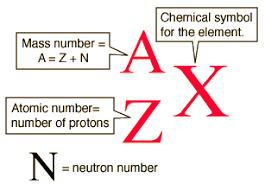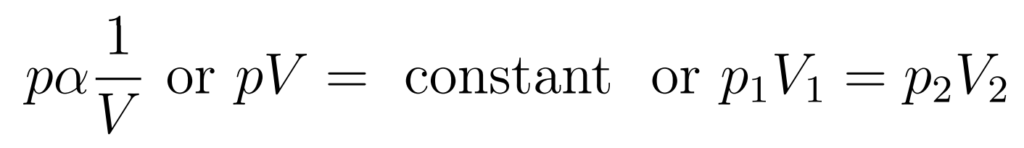Kinetic Theory of Gases - Topic 3.2
Bite-sized Kinetic Theory of Gases Study Notes for IB Physics HL/SL
Table of Contents

Key point
- The number of particles in a mole is equal to the Avogadro Constant
- A ‘particle’ can mean an atom or a molecule
- The number of particles in a mole is N A
Formula Booklet
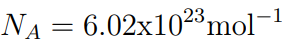
Formula Booklet
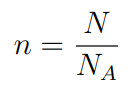
If a substance contains N particles, then the number of moles ‘n‘ is given by this formula.
- n = number of moles
- N = Number of particles
- NA = Avogadro’s Constant
The atomic mass unit (1u) is defined as 1/12 of the mass of a carbon-12 atom.
- The mass per one neutron is 1 mole
- The mass per one proton is 1 mole
- Molar mass is the sum of all atomic masses of a molecule
Formula Booklet
Unified atomic mass unit
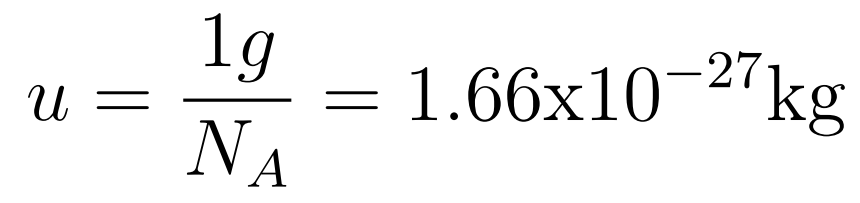
Key point
- Pressure is the normal force applied per unit area
- The force is applied normal to the area
- If the force is given by with an angle, then find the vertical component of the angle
- P = Pressure
- F = Force
- A = Area
- Unit: Newton per square metre, Nm-2 = pascal, Pa
- Can also be used: atmospere, atm = 1.013 x 105 Pa
Formula Booklet
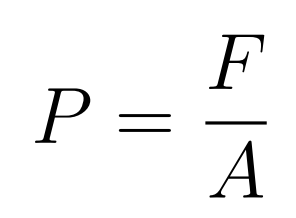
Key points
- An ideal gas is a theoretical model of a real gas
- A real gas can be approximated by an ideal gas at low density (low pressure, high temperature)
An Ideal Gas is a theoretical model of a gas. It is defined by the following assumptions:
- The molecules are point particles – with negligible volume
- The molecules obey the laws of mechanics
- An ideal gas can not be liquefied or solidified
- Molecules have a variety of speeds and move randomly
- There are no forces between the molecules except when the molecules collide
- The duration of a collision is negligible compared to the time between collisions
- The collisions of the molecules with each other and with the container walls are elastic
At very low density (low pressure, high temperature) a real gas will generally behave like an ideal gas.
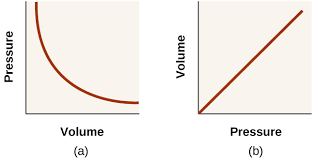
Key point
- Pressure is inversely proportional to volume
The Pressure-Volume Law states that at constant temperature and with a fixed quantity of gas, pressure is inversely proportional to volume.
- This relationship is also known as Boyle’s Law
- The curve is isothermal – the temperature at all points on the curve is the same
- The product pV is the same for all points on the curve
Key point
- Volume increases uniformly with temperature
The Volume-Temperature Law states that when temperature is expressed in kelvin, then at constant temperature volume increases uniformly with temperature.
- Charle’s Law
Not in Formula Booklet but important
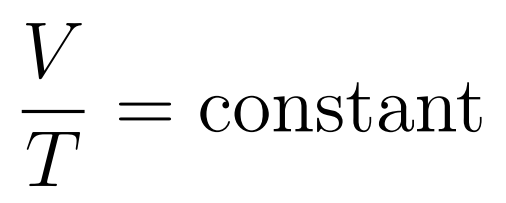
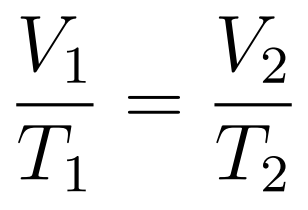
Key point
- Pressure increases uniformly with increasing temperature
The Pressure-Temperature Law states that when temperature is expressed in kelvin and volume is constant, then pressure increases uniformly with increasing temperature.
- Gay-Lussac’s Law or Amonton’s Law
Not in Formula Booklet but important
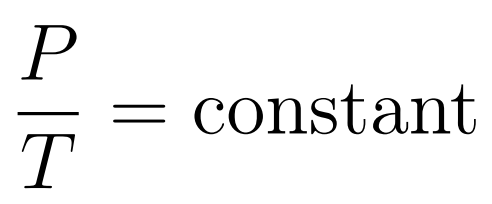
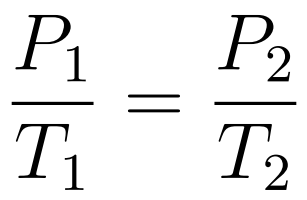
Key point
- The Gas constant (R) is a universal constant
The Equation of state of an Ideal Gas relates Volume, Pressure, Temperature, and the number of moles of a gas. .
- R is the universal gas constant
- p = pressure
- V = Volume
- n = number of moles
- T = temperature
- Through the introduction of the universal gas constant one can now calculate for the missing quantities (with the help of the given quantities)
Formula Booklet
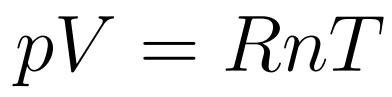
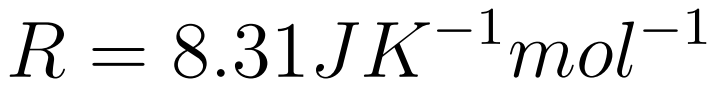
Key points
- Calculate the Kinetic Energy of a gas
- The Internal Energy of an ideal gas consists only of its kinetic energy
The molecules of a gas move randomly with a range of speeds.
- kb = Boltzmann constant
- T = Temperature
- EK = Kinetic Energy
Formula Booklet
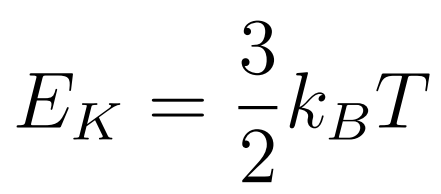
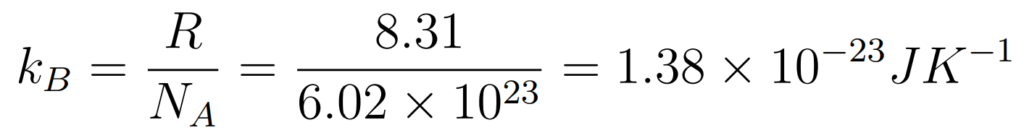
Not in Formula Booklet but important
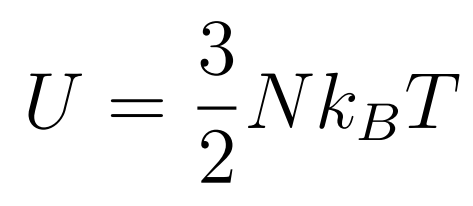
The Internal Energy of an ideal gas consists only of the random kinetic energy of its molecules
- N = Number of Molecules
- kB = Boltzmann constant
- T = Temperature
- Remember the basic rules that characterize an ideal gas
Worksheet - Kinetic Molecular Theory of Gases
Worksheet with Answers
Questions and Answers
Worksheet with Answers 2.0
Questions and Answers
PPT (PDF) - Kinetic Molecular Theory of Gases
Subscribe to the Inertia Newsletter
IB News, Covid-19 Updates, Deadlines, Tips and Tricks, and Hundreds of Free Resources are Awaiting You!
Features
- Study Notes
- Thousands of IB Questions
- Detailed Answers
- Ask-A-Question System