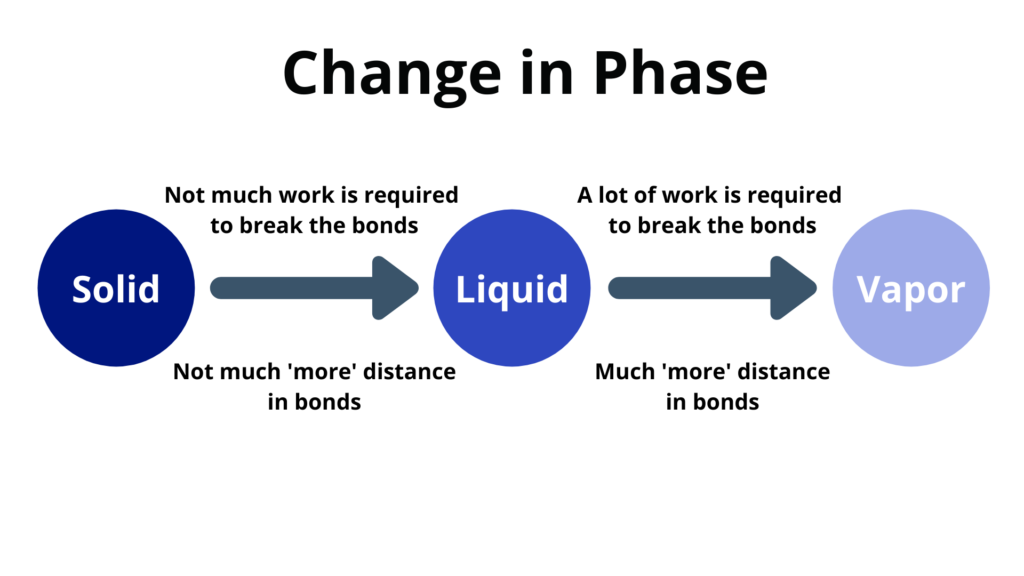
Change of Phase
Key point
- When heat is provided to a body it doesn’t mean that the body will change its temperature – it may also change its phase
Change of phase happens at a constant temperature.
- Melting – when a solid changes to a liquid
- Freezing – when a liquid changes to a solid
- Vaporization – when a liquid changes to a vapor
- Condensation – when a vapor changes into a liquid
- The heat energy goes into the potential energy instead of the kinetic energy
Formula Booklet
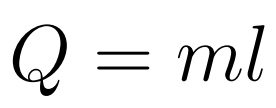
Specific Latent Heat (l) is defined as the amount of energy required to change the phase of a unit mass at a constant temperature.
- Q = Energy/Heat
- l = Specific latent heat
- m = mass
Subscribe to the Inertia Newsletter
IB News, Covid-19 Updates, Deadlines, Tips and Tricks, and Hundreds of Free Resources are Awaiting You!
Features
- Study Notes
- Thousands of IB Questions
- Detailed Answers
- Ask-A-Question System


