Heat
Key point
- Heat is energy that is transferred between bodies
Heat is the energy that is transferred from one body to another body as a result of a difference in temperature.
- Each Molecule consists of particles
- Kinetic Energy – the speed of vibration
- Potential Energy – the separation between the particles
- Internal Energy – the sum of Kinetic Energy and the inter-particle potential energy
- Heat transferred between a hot and a cold body increases the internal energy of the cold body
- Work done on particles increases the potential energy
- Thus the internal energy can change due to work performed or heat transfer
- Internal Energy, heat, and work are all measured in Joules (J)
- Temperature is a measure of the random kinetic energy of a molecule
Formula Booklet
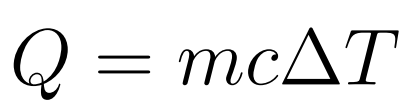
Specific Heat Capacity is the amount of energy needed in order to increase a unit of mass of the object by one kelvin.
- Q = Energy/Heat
- m = mass
- c = specific heat capacity
- ΔT = change in Temperature
Subscribe to the Inertia Newsletter
IB News, Covid-19 Updates, Deadlines, Tips and Tricks, and Hundreds of Free Resources are Awaiting You!

